

7 Junvon Close, Ogbaku Imo State

 By Staff
By Staff
 No Comments
No Comments  3 minutes
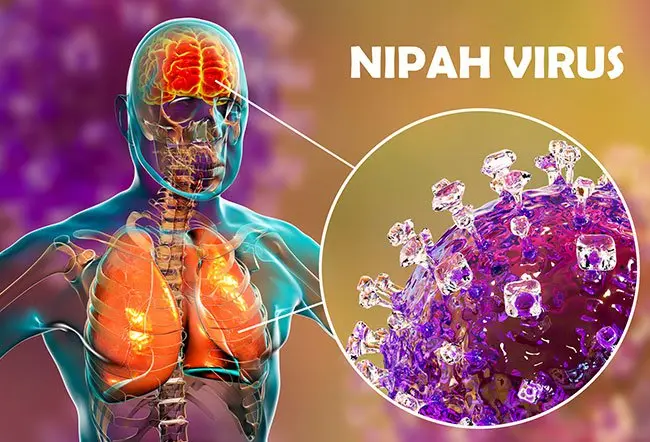
3 minutes Nipah virus (NiV) is a rare but serious viral infection that can cause severe illness in humans and animals. First identified in 1998–1999 during an outbreak among pig farmers in Malaysia, Nipah virus has since been recognized as a priority pathogen due to its high fatality rate and potential to cause outbreaks. While cases are uncommon, awareness and prevention are critical, especially in regions where the virus has previously occurred.
Nipah virus belongs to the Henipavirus genus and is naturally carried by fruit bats (flying foxes of the Pteropus species). These bats often show no symptoms but can transmit the virus to other animals and humans.
Humans can become infected through direct or indirect contact with infected animals, contaminated food, or from other infected people.
Nipah virus can spread through:
Healthcare settings and family caregiving environments are particularly high-risk if proper infection control measures are not followed.
Symptoms usually appear 4–14 days after exposure but may take longer in some cases. They can range from mild to severe and include:
In severe cases, Nipah virus infection can be fatal. Survivors may experience long-term neurological complications.
Nipah virus infection is diagnosed through laboratory testing of blood, urine, throat swabs, or cerebrospinal fluid. Tests are typically performed in specialized laboratories due to the virus’s high-risk nature.
Early diagnosis is essential for patient care and to prevent further spread.
Currently, there is no specific antiviral treatment or approved vaccine for Nipah virus. Management focuses on:
Early medical attention significantly improves outcomes.
Preventing Nipah virus relies heavily on public health measures and individual awareness:
Public education and surveillance play a major role in reducing outbreaks.
Nipah virus is classified by the World Health Organization as a high-priority pathogen because:
Ongoing research is focused on vaccine development, antiviral therapies, and improved outbreak response.
Although rare, Nipah virus is a serious infectious disease that requires vigilance, especially in at-risk regions. Awareness, early detection, and preventive practices are the most effective tools currently available to protect individuals and communities.
Staying informed and following public health guidance can help reduce the risk of infection and save lives.
This article is for educational purposes only and should not replace professional medical advice.
| S | M | T | W | T | F | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |